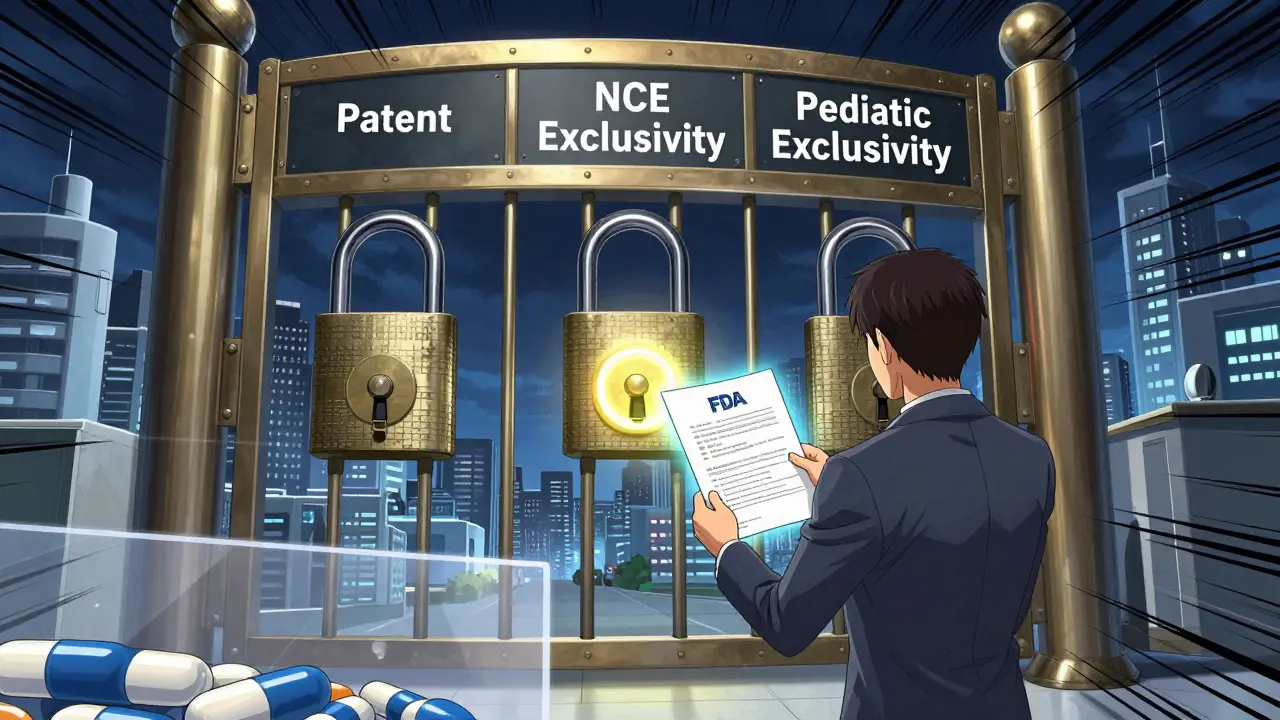
Pediatric Exclusivity: How the FDA Extends Market Protection Without Touching Patents
Pediatric exclusivity lets the FDA block generic drugs for six extra months after patents expire - not by extending patents, but by adding a regulatory hold. Here's how it works, who benefits, and why it matters.
Read More